The world of chemistry is full of mysteries, and one of the most fascinating aspects is the structure of molecules. A single molecule can hold so much information about its properties, reactions, and interactions with other molecules. In this blog post, we’ll be exploring a specific Lewis structure that might look simple at first glance but holds many secrets: CCOOHhhh.
The Mysterious Atom
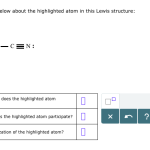
As you gaze upon the Lewis structure, your mind might wander to the many questions it raises. What are the roles of each atom? How do they interact with one another? And what secrets lie hidden in this seemingly simple molecule?
Let’s Start with the Basics
The Lewis structure for CCOOHhhh may look like a jumbled mess, but let’s take a step back and examine it more closely. At its core is the carbon atom (C), surrounded by oxygen (O) and hydrogen (H) atoms. This molecule is known as a carboxylic acid, a crucial component in many biological processes.
One of the key points to note about this molecule is that it has both polar and non-polar regions. The carboxyl group (-COOH) is highly polar, with the oxygen atom’s electronegativity drawing the electrons towards itself, making it slightly negative. Meanwhile, the alkyl chain (hhh) is relatively non-polar.
This contrast between polar and non-polar regions creates an interesting phenomenon known as hydrophilic-hydrophobic interactions. The polar carboxyl group can form hydrogen bonds with water molecules, while the non-polar alkyl chain repels water due to its hydrophobic nature. This interplay has significant implications for the molecule’s behavior in different environments.
In our next section, we’ll delve deeper into these hydrophilic-hydrophobic interactions and explore how they impact the molecule’s properties and reactions.
The world of chemistry is full of mysteries, and one of the most fascinating aspects is the structure of molecules. A single molecule can hold so much information about its properties, reactions, and interactions with other molecules. In this blog post, we’ll be exploring a specific Lewis structure that might look simple at first glance but holds many secrets: CCOOHhhh.
The Mysterious Atom
As you gaze upon the Lewis structure, your mind might wander to the many questions it raises. What are the roles of each atom? How do they interact with one another? And what secrets lie hidden in this seemingly simple molecule?
Let’s Start with the Basics
The Lewis structure for CCOOHhhh may look like a jumbled mess, but let’s take a step back and examine it more closely. At its core is the carbon atom (C), surrounded by oxygen (O) and hydrogen (H) atoms. This molecule is known as a carboxylic acid, a crucial component in many biological processes.
One of the key points to note about this molecule is that it has both polar and non-polar regions. The carboxyl group (-COOH) is highly polar, with the oxygen atom’s electronegativity drawing the electrons towards itself, making it slightly negative. Meanwhile, the alkyl chain (hhh) is relatively non-polar.
This contrast between polar and non-polar regions creates an interesting phenomenon known as hydrophilic-hydrophobic interactions. The polar carboxyl group can form hydrogen bonds with water molecules, while the non-polar alkyl chain repels water due to its hydrophobic nature. This interplay has significant implications for the molecule’s behavior in different environments.
To further understand these interactions, let’s consider a few examples. For instance, when a carboxylic acid like CCOOHhhh is dissolved in water, the polar carboxyl group can form hydrogen bonds with surrounding water molecules, allowing the molecule to dissolve and interact with its environment. On the other hand, if the same molecule were placed in a non-polar solvent like hexane, the hydrophobic alkyl chain would dominate, causing the molecule to precipitate out of solution.
This concept is crucial in understanding biological processes, as it explains how molecules can selectively interact with specific environments. For instance, enzymes that are highly specific for certain substrates can be influenced by these interactions, allowing them to bind and catalyze reactions efficiently.
In our next section, we’ll delve deeper into the implications of hydrophilic-hydrophobic interactions on the molecule’s properties and reactions, exploring how they impact its behavior in different environments. We’ll also examine how these principles apply to other molecules and systems, highlighting their significance in understanding biological processes.
Hydrophilic-Hydrophobic Interactions: What’s Next?
In our next section, we’ll explore the implications of hydrophilic-hydrophobic interactions on the molecule’s properties and reactions. Stay tuned to learn more about how this fascinating concept impacts our understanding of biological processes.
Get Expert Help with Your Chemistry Questions
Don’t struggle to understand Lewis structures and molecular bonding. Our experts are here to guide you through complex chemistry concepts.
Start chatAs we’ve explored the Lewis structure of CCOOHhhh, we’ve uncovered the intricate relationships between its atoms and their polar and non-polar regions. This carboxylic acid molecule is more than just a simple combination of atoms – it’s a dynamic system with properties that shape its behavior in different environments.
So what secrets does this molecule hold? For starters, its hydrophilic-hydrophobic interactions play a crucial role in determining its solubility and reactivity. The polar carboxyl group can form hydrogen bonds with water molecules, making the molecule soluble in aqueous solutions. Meanwhile, the non-polar alkyl chain repels water, reducing the molecule’s overall solubility.
This interplay has significant implications for the molecule’s behavior in biological systems. Carboxylic acids like CCOOHhhh are essential components of many biomolecules, including amino acids, fatty acids, and cholesterol. Their polar regions allow them to form hydrogen bonds with water and other molecules, influencing their interactions and functions within cells.
In conclusion, the Lewis structure of CCOOHhhh may seem simple at first glance, but it holds a wealth of information about its properties, reactions, and interactions. By examining this molecule’s polar and non-polar regions, we’ve gained insight into its behavior in different environments and its importance in biological systems. Whether you’re a chemistry enthusiast or just curious about the world around us, I hope this blog post has inspired you to explore the fascinating realm of molecular structures.
And so, as we conclude our journey through the world of molecules, remember that even the simplest-looking structures can hold complex secrets waiting to be uncovered. Who knows what mysteries lie hidden in the Lewis structures yet to come? The world of chemistry is full of surprises, and it’s up to us to explore and discover them.
Black beans nutrition fact: Are you looking for a nutritious addition to your diet? Black beans are packed with protein, fiber, and vitamins. Discover the amazing benefits of incorporating black beans into your meals.
Symptoms of fatty liver due to alcohol consumption: Are you a heavy drinker and concerned about the impact on your health? Fatty liver disease is a common consequence of excessive alcohol consumption. Learn the warning signs and take steps to protect your liver.