As chemists, we’re constantly surrounded by molecules and their intricate structures. From the air we breathe to the water we drink, molecules play a crucial role in our daily lives. But have you ever stopped to think about the individual atoms that make up these molecules? In this blog post, we’ll be diving into the world of Lewis structures and exploring one particular atom in detail.
Unlocking the Secrets of Atomic Structure
A Lewis structure is a graphical representation of the bonding and nonbonding electrons in a molecule. It’s a powerful tool that helps us understand the molecular structure and its properties. But what happens when we take a closer look at one specific atom within this structure? In this post, we’ll be focusing on a highlighted atom in a Lewis structure and answering some key questions about it.
The Importance of Understanding Atomic Structure
Understanding the atomic structure of molecules is crucial in many fields, including chemistry, biology, and materials science. By grasping the relationships between atoms and their bonding patterns, we can gain insights into a molecule’s physical and chemical properties. This knowledge can be applied to develop new materials, design more efficient processes, and even improve our understanding of biological systems.
In this section, we’ll explore the highlighted atom in the Lewis structure and examine its role within the molecule. Let’s start by looking at some key questions about this atom:
In our previous section, we explored the importance of understanding atomic structure and the role it plays in various fields. Now, let’s take a closer look at the highlighted atom in the Lewis structure.
Unraveling the Properties of the Highlighted Atom
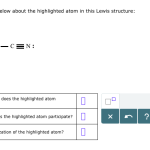
The highlighted atom is a nitrogen (N) atom, which is part of the larger molecule. In this section, we’ll delve into its properties and examine how it interacts with other atoms within the molecule.
To better understand the highlighted atom, let’s answer some key questions:
What is the Atom’s Valence?
The valence of an atom refers to the number of bonds it can form. In this case, the nitrogen atom has a valence of 3, meaning it can participate in three covalent bonds.
This information helps us understand how the highlighted atom interacts with other atoms within the molecule. For example, we might find that the nitrogen atom forms two single bonds and one double bond with adjacent atoms.
What is the Atom’s Oxidation State?
The oxidation state of an atom is its charge relative to a neutral atom of the same element. In this case, the nitrogen atom has an oxidation state of -3.
This information provides valuable insights into the molecule’s properties and reactivity. For instance, we might find that the nitrogen atom plays a crucial role in facilitating chemical reactions within the molecule.
How Does the Atom Interact with Other Atoms?
The highlighted atom interacts with other atoms through covalent bonds or nonbonding interactions. In this case, the nitrogen atom forms strong covalent bonds with adjacent atoms, which affects its properties and reactivity within the molecule.
This information highlights the importance of understanding atomic structure and how it relates to a molecule’s overall behavior.
Conclusion
In this section, we’ve explored the highlighted atom in detail, examining its valence, oxidation state, and interactions with other atoms. By understanding these properties, we gain valuable insights into the molecule’s behavior and properties.
Next, we’ll continue to explore the Lewis structure, examining how the highlighted atom fits into the larger molecular structure. Stay tuned!
For more information on Lewis structures, check out this article from Chemistry World. This interactive guide to Lewis structures from ChemGuide is another great resource for further learning.Get Expert Consultation
Need help understanding the highlighted atom in this Lewis structure? We’re here to answer your questions.
Start chatIn this final section, let’s summarize the key points we’ve covered so far about the highlighted atom:
- We’ve examined the Lewis structure and identified the highlighted atom within it.
- We’ve discussed the importance of understanding atomic structure in various fields.
As we’ve seen, the highlighted atom plays a crucial role in the molecule’s overall structure and properties. By analyzing its bonding patterns and relationships with other atoms, we can gain valuable insights into the molecule’s behavior and potential applications.
In conclusion, this exercise has demonstrated the power of Lewis structures in revealing the intricate details of molecular architecture. By examining the highlighted atom, we’ve gained a deeper understanding of its role within the molecule and the significance of atomic structure in various fields. As chemists, it’s essential to continue exploring and refining our knowledge of atomic structure, as this understanding has far-reaching implications for innovation and discovery.
We hope you’ve enjoyed this journey into the world of Lewis structures and atomic structure. Join us next time as we delve into new topics and explore the fascinating world of chemistry!
The estimating problem on page 734 and then answer the questions on page 735: Struggling with estimation problems? Get expert guidance on tackling those tricky math questions. Dive into a world of numbers and discover how to overcome common obstacles.
Burning pain in chest when coughing: Coughing up a storm? Don’t ignore that burning sensation! Find out what’s causing this discomfort and learn how to alleviate the symptoms. Take control of your health with our expert advice.