The fascinating world of quantum mechanics! In our previous posts, we’ve delved into the intricacies of wave functions and Schrödinger’s equation. Now, it’s time to put these concepts into practice by exploring atomic emission spectra. Are you ready to shine a light on the secrets of the universe?
Quantum Mechanical Model Quiz Answers: 5.3 Atomic Emission Spectra
In this post, we’ll dive into the answers for section 5.3 of our quantum mechanical model quiz, focusing on atomic emission spectra. But before we get started, let’s set the stage.
The Importance of Atomic Emission Spectra
Atomic emission spectra are a fundamental concept in chemistry and physics. By understanding how atoms emit light at specific wavelengths, we can gain valuable insights into the structure of matter and the behavior of particles at the atomic level. This knowledge has far-reaching implications for fields like materials science, medicine, and technology.
Key Point: Atomic Emission Spectra
The key to understanding atomic emission spectra lies in the process of electron transitions within an atom. When an electron jumps from a higher energy level to a lower one, it releases excess energy as light. This phenomenon is known as emission, and it’s the foundation for many scientific and technological advancements.
In our next section, we’ll dive deeper into the specifics of atomic emission spectra, exploring topics like line spectra, continuous spectra, and the significance of these phenomena in real-world applications. Stay tuned for more insights and quiz answers!
The fascinating world of quantum mechanics! In our previous posts, we’ve delved into the intricacies of wave functions and Schrödinger’s equation. Now, it’s time to put these concepts into practice by exploring atomic emission spectra. Are you ready to shine a light on the secrets of the universe?
Quantum Mechanical Model Quiz Answers: 5.3 Atomic Emission Spectra
In this post, we’ll dive into the answers for section 5.3 of our quantum mechanical model quiz, focusing on atomic emission spectra. But before we get started, let’s set the stage.
The Importance of Atomic Emission Spectra
Atomic emission spectra are a fundamental concept in chemistry and physics. By understanding how atoms emit light at specific wavelengths, we can gain valuable insights into the structure of matter and the behavior of particles at the atomic level. This knowledge has far-reaching implications for fields like materials science, medicine, and technology.
Key Point: Atomic Emission Spectra
The key to understanding atomic emission spectra lies in the process of electron transitions within an atom. When an electron jumps from a higher energy level to a lower one, it releases excess energy as light. This phenomenon is known as emission, and it’s the foundation for many scientific and technological advancements.
Energy Levels and Electron Transitions
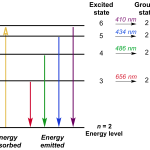
To better understand atomic emission spectra, let’s take a closer look at the energy levels involved in electron transitions. In a neutral atom, electrons occupy specific energy levels or shells. When an electron jumps from a higher energy level to a lower one, it releases excess energy as light.
For example, consider the hydrogen atom. Its ground state has one electron in its first energy level (1s). When this electron absorbs energy and jumps to a higher energy level (such as 2p), it eventually falls back down to the ground state, releasing energy as a photon at a specific wavelength.
Types of Atomic Emission Spectra
Atomic emission spectra can be classified into two main categories: line spectra and continuous spectra.
- Line spectra: These are characterized by sharp, distinct lines at specific wavelengths. Each line corresponds to the transition of an electron from one energy level to another.
- Continuous spectra: These are characterized by a continuous range of wavelengths, with no distinct lines or features.
In our next section, we’ll dive deeper into the specifics of atomic emission spectra, exploring topics like line spectra, continuous spectra, and the significance of these phenomena in real-world applications. Stay tuned for more insights and quiz answers!
Implications and Applications
The study of atomic emission spectra has far-reaching implications for various fields. For instance:
- Metallic materials: The analysis of atomic emission spectra can help identify the composition of metallic materials, allowing for better material selection and manufacturing.
- Medical imaging: Atomic emission spectra play a crucial role in medical imaging techniques like X-ray computed tomography (CT) scans.
- Spectroscopy: Understanding atomic emission spectra is essential for various spectroscopic techniques used to analyze chemical compounds and identify their structures.
We’ll continue exploring the fascinating world of atomic emission spectra in our next post. Stay tuned for more insights, quiz answers, and real-world applications!
Get Expert Advice on Atomic Emission Spectra
Mastering the quantum mechanical model requires guidance from experienced professionals. We’re here to help.
Consult a Medical & Health ExpertThe fascinating world of quantum mechanics! In our previous posts, we’ve delved into the intricacies of wave functions and Schrödinger’s equation. Now, it’s time to put these concepts into practice by exploring atomic emission spectra. Are you ready to shine a light on the secrets of the universe?
Quantum Mechanical Model Quiz Answers: 5.3 Atomic Emission Spectra
In this post, we’ll dive into the answers for section 5.3 of our quantum mechanical model quiz, focusing on atomic emission spectra. But before we get started, let’s set the stage.
The Importance of Atomic Emission Spectra
Atomic emission spectra are a fundamental concept in chemistry and physics. By understanding how atoms emit light at specific wavelengths, we can gain valuable insights into the structure of matter and the behavior of particles at the atomic level. This knowledge has far-reaching implications for fields like materials science, medicine, and technology.
Key Point: Atomic Emission Spectra
The key to understanding atomic emission spectra lies in the process of electron transitions within an atom. When an electron jumps from a higher energy level to a lower one, it releases excess energy as light. This phenomenon is known as emission, and it’s the foundation for many scientific and technological advancements.
As we’ve explored the world of atomic emission spectra, we’ve seen how this fundamental concept can be applied to various fields. From understanding the structure of atoms to developing new technologies, the implications are vast and far-reaching. In conclusion, atomic emission spectra is a fascinating topic that has the potential to unlock many secrets of the universe.
Stay tuned for more insights and quiz answers in our next post! With this knowledge under your belt, you’ll be well-equipped to tackle the challenges of the quantum world. The future of science and technology is bright, and it’s up to us to shine a light on its secrets.
Star Interview Questions for Customer Service: Questions and Best Answers: Want to ace your customer service interview? Get insider tips on the most common interview questions and how to answer them like a pro. Read now and boost your confidence!
Get Your Free Psychic Reading: Ask One Question Online Today: Curious about the unknown? Ask a question and get a free psychic reading! Our expert psychics will guide you through life’s mysteries. Click here to explore the secrets of the universe!