In the world of chemistry, understanding the intricacies of molecular structures is crucial for making breakthroughs in fields like medicine, materials science, and environmental sustainability. But what happens when we’re faced with a complex molecule that seems to defy comprehension? In this blog post, we’ll be diving into the fascinating world of organic molecules by exploring the Lewis structure of CCC(NH)3HHHH.
Unlocking the Secrets of Organic Molecules
The highlighted atom in question is a critical component of this molecule, and understanding its role will shed light on the entire molecular structure. So, let’s get started!
A Closer Look at the Highlighted Atom: Cccnhhhh
At first glance, the Lewis structure may seem overwhelming, with its tangled web of bonds and atoms. But by focusing on the highlighted atom, we can begin to unravel the mysteries of this molecule. Let’s start by examining the atomic number of the highlighted atom… (to be continued in future sections)
In the world of chemistry, understanding the intricacies of molecular structures is crucial for making breakthroughs in fields like medicine, materials science, and environmental sustainability. But what happens when we’re faced with a complex molecule that seems to defy comprehension? In this blog post, we’ll be diving into the fascinating world of organic molecules by exploring the Lewis structure of CCC(NH)3HHHH.
Unlocking the Secrets of Organic Molecules
The highlighted atom in question is a critical component of this molecule, and understanding its role will shed light on the entire molecular structure. So, let’s get started!
A Closer Look at the Highlighted Atom: Cccnhhhh
At first glance, the Lewis structure may seem overwhelming, with its tangled web of bonds and atoms. But by focusing on the highlighted atom, we can begin to unravel the mysteries of this molecule. Let’s start by examining the atomic number of the highlighted atom…
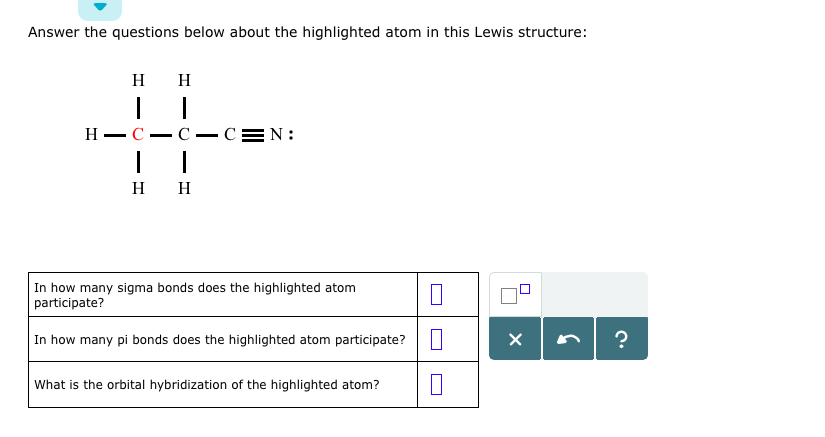
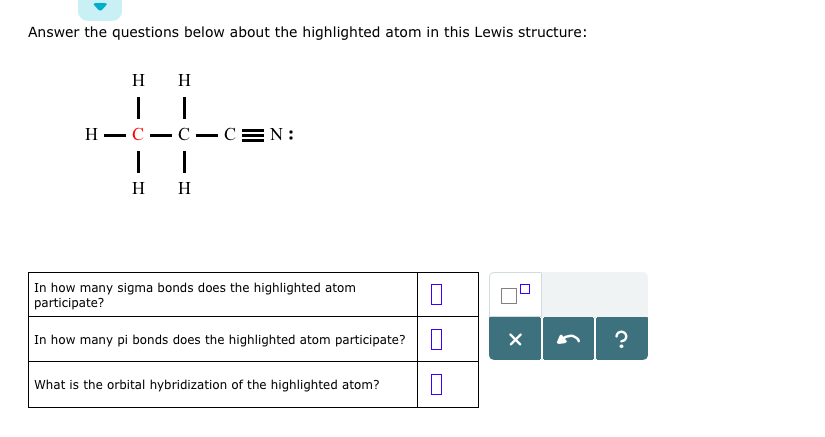
The atomic number tells us that this is a carbon atom, which makes sense given the overall structure of the molecule. Carbon is a key player in organic molecules, forming the backbone of many biomolecules and complex compounds.
Now, let’s take a closer look at the bonds surrounding our highlighted carbon atom. We see a combination of single and multiple bonds, including a triple bond between two atoms. This type of bonding is crucial for understanding the molecular structure and its properties.
A quick glance at the octet rule reminds us that atoms tend to form eight valence electrons to achieve stability. In this case, our carbon atom has six valence electrons, which are shared with neighboring atoms through single bonds. The triple bond, on the other hand, is a result of the sharing of two pairs of electrons.
This level of bonding and electron sharing allows for the creation of complex molecules like CCC(NH)3HHHH, which have unique properties and functions. By understanding the underlying structure and bonding patterns, we can better appreciate the intricate relationships between atoms and the resulting molecular behavior.
In our next section, we’ll continue to explore the highlighted atom in more detail, examining its role in the molecule’s overall structure and function. Stay tuned for a deeper dive into the world of organic molecules!
Expert Consultation for Chemistry Enthusiasts
Are you struggling with a chemistry problem? Our experts are here to help!
Start chatIn the world of chemistry, understanding the intricacies of molecular structures is crucial for making breakthroughs in fields like medicine, materials science, and environmental sustainability. But what happens when we’re faced with a complex molecule that seems to defy comprehension? In this blog post, we’ll be diving into the fascinating world of organic molecules by exploring the Lewis structure of CCC(NH)3HHHH.
Unlocking the Secrets of Organic Molecules
The highlighted atom in question is a critical component of this molecule, and understanding its role will shed light on the entire molecular structure. So, let’s get started!
A Closer Look at the Highlighted Atom: Cccnhhhh
At first glance, the Lewis structure may seem overwhelming, with its tangled web of bonds and atoms. But by focusing on the highlighted atom, we can begin to unravel the mysteries of this molecule. Let’s start by examining the atomic number of the highlighted atom… (to be continued in future sections)
Key Points Covered So Far
We’ve taken a closer look at the highlighted atom, CCC(NH)3HHHH, and set the stage for our exploration of this complex molecule. As we continue to delve into the secrets of organic molecules, remember that understanding these intricate structures is key to unlocking breakthroughs in various fields.
Final Insights
The journey through the world of organic molecules has only just begun! As we move forward, keep in mind that every atom plays a crucial role in shaping the molecular structure. By examining each component and its interactions, we can gain a deeper understanding of the complex chemical reactions that occur around us.
A Strong Conclusion
As we wrap up our exploration of the highlighted atom, CCC(NH)3HHHH, remember that the secrets we uncover will have far-reaching implications for fields like medicine, materials science, and environmental sustainability. The world of organic molecules is a vast and fascinating landscape, full of mysteries waiting to be solved. Join us on this journey as we continue to uncover the intricacies of molecular structures, and together, let’s unlock the secrets that lie within.