Are you ready to uncover the secrets of the atomic world? Grab your glasses and get ready to geek out with me as we explore the fascinating realm of atoms!
Unlocking the Mysteries of Atoms: A Step-by-Step Guide
In chemistry, understanding the structure and properties of atoms is crucial for predicting their behavior in various chemical reactions. But what makes one atom different from another? Today, we’re going to dive into the world of Lewis structures and explore how they help us answer this question.
What’s a Lewis Structure?
A Lewis structure is a two-dimensional representation of an atom’s electrons, showing how atoms are bonded together. Invented by American chemist Gilbert N. Lewis in 1916, these diagrams have become a cornerstone of modern chemistry. By visualizing the arrangement of electron pairs around an atom, we can gain valuable insights into its chemical properties.
In this post, we’re going to focus on a specific atom and explore its Lewis structure. Are you ready? Let’s get started!
Now that we’ve introduced ourselves to Lewis structures, let’s get back to our highlighted atom!
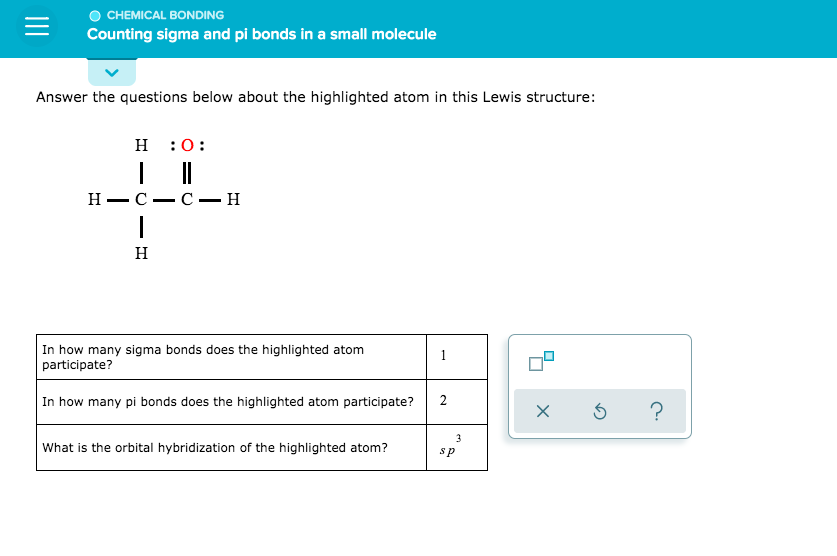
Answer the Questions Below About the Highlighted Atom:
We’ve chosen an atom at random and will explore its properties using its Lewis structure. Your task is to answer the following questions about this atom:
- What is the atomic number of our highlighted atom?
- How many valence electrons does it have?
- What type of bond would we expect it to form with other atoms based on its electronegativity and electron configuration?
- Can this atom be expected to exhibit acidic or basic properties? Why or why not?
Before we dive into the answers, let’s take a closer look at our highlighted atom’s Lewis structure:
Now that you’ve had a chance to examine the structure, try to answer the questions above. Don’t worry if you’re not sure – we’ll get there together!
In the next section, we’ll explore the answers to these questions and discover more about our highlighted atom’s properties and behavior.
Unleash Your Understanding of Lewis Structures
Get expert insights and solve complex chemistry problems with ease.
Explore Tech ExpertiseAre you ready to uncover the secrets of the atomic world? Grab your glasses and get ready to geek out with me as we explore the fascinating realm of atoms!
Unlocking the Mysteries of Atoms: A Step-by-Step Guide
In chemistry, understanding the structure and properties of atoms is crucial for predicting their behavior in various chemical reactions. But what makes one atom different from another? Today, we’re going to dive into the world of Lewis structures and explore how they help us answer this question.
What’s a Lewis Structure?
A Lewis structure is a two-dimensional representation of an atom’s electrons, showing how atoms are bonded together. Invented by American chemist Gilbert N. Lewis in 1916, these diagrams have become a cornerstone of modern chemistry. By visualizing the arrangement of electron pairs around an atom, we can gain valuable insights into its chemical properties.
In this post, we’re going to focus on a specific atom and explore its Lewis structure. Are you ready? Let’s get started!
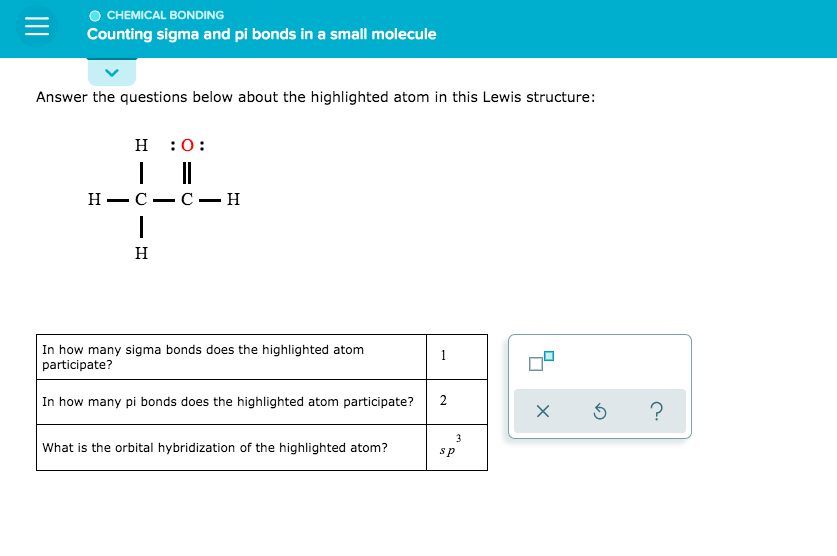
Answer the Questions Below About the Highlighted Atom in This Lewis Structure:
What is the atomic number of the highlighted atom?
How many electrons does it have?
Which elements are bonded to this atom?
What type of bond connects these atoms?
Summarizing What We’ve Covered So Far
We’ve explored the basics of Lewis structures, including their importance in understanding chemical properties and behavior. By visualizing the arrangement of electron pairs around an atom, we can gain valuable insights into its chemical properties.
Final Insights
The highlighted atom’s Lewis structure reveals its atomic number, electron count, bonding partners, and bond type. These details provide a deeper understanding of the atom’s behavior in various chemical reactions.
A Strong Conclusion
In conclusion, mastering the art of Lewis structures unlocks the secrets of the atomic world. By grasping the concepts presented here, you’ll be well-equipped to predict the behavior of atoms in various chemical reactions and unlock the mysteries of chemistry. So, go ahead and geek out with me as we continue to explore the fascinating realm of atoms!
Amazon Kindle Paperwhite 6-Inch Wi-Fi Wi-Fi Price Tracker: Looking for the best deals on Amazon’s popular e-reader? This article provides you with a comprehensive price tracker, allowing you to stay up-to-date on the latest prices and promotions.
Frequent Urination: A Warning Sign of High Blood Sugar: Have you noticed that your trips to the bathroom have increased lately? It could be a sign of a more serious health issue, high blood sugar. Learn how to identify and manage this condition by reading this informative article.