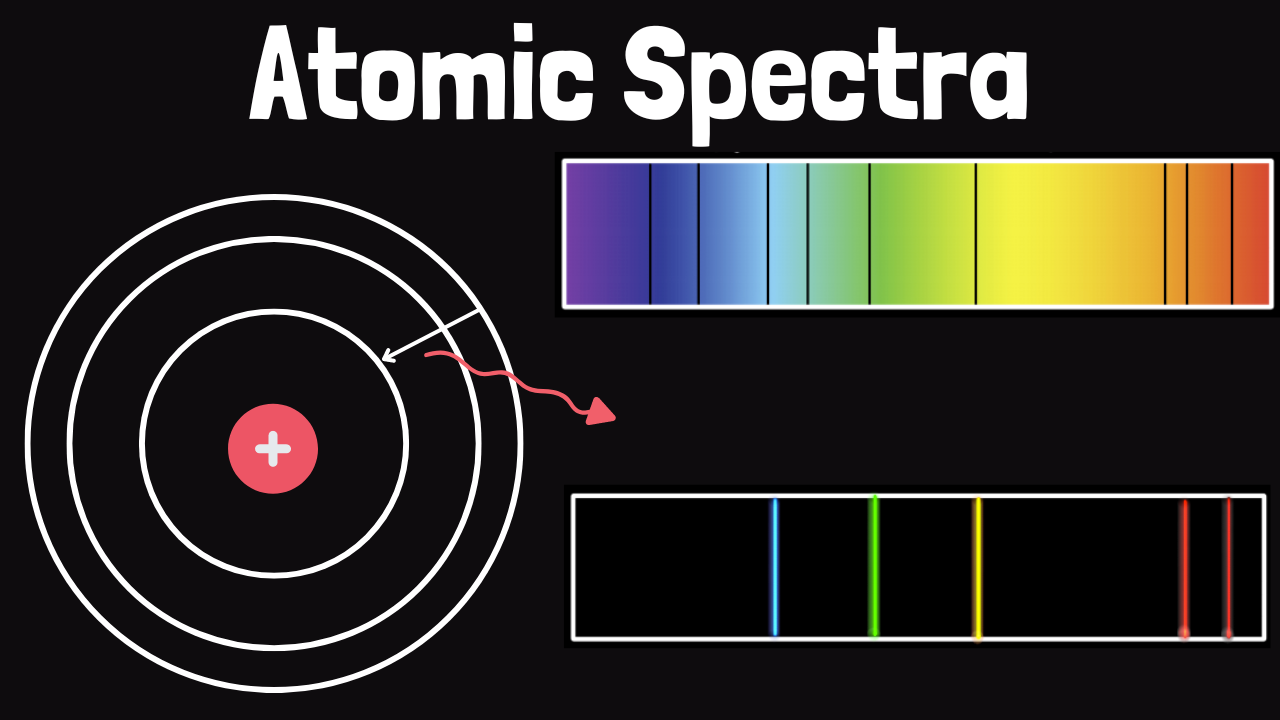
Have you ever gazed up at the night sky, mesmerized by the swirling colors of the aurora borealis? Or perhaps you’ve marveled at the bright glow of neon signs or the warm light of a candle flame. These breathtaking displays of color and light are all thanks to atomic emission spectra!
Unveiling the Secrets of Atomic Emission Spectra
In this post, we’ll delve into the fascinating world of 5.3 atomic emission spectra and explore how they reveal the secrets of the quantum mechanical model.
A Closer Look at Atomic Emission Spectra
So, what exactly is an atomic emission spectrum? In simple terms, it’s the unique “finger print” left by atoms as they transition from a higher energy state to a lower one. This process releases specific amounts of energy in the form of light, which we can observe and analyze.
But why do atomic emission spectra matter? They’re crucial for understanding the behavior of atoms and molecules at the quantum level. By studying these spectra, scientists can gain valuable insights into the properties and interactions of atoms, leading to breakthroughs in fields like chemistry, materials science, and beyond!
The Quantum Mechanical Model: Unlocking the Secrets of Atomic Emission Spectra
Now that we’ve set the stage for atomic emission spectra, let’s dive deeper into the quantum mechanical model. In the next section, we’ll explore how this model helps us make sense of these spectral patterns and what they reveal about the behavior of atoms at the atomic level.
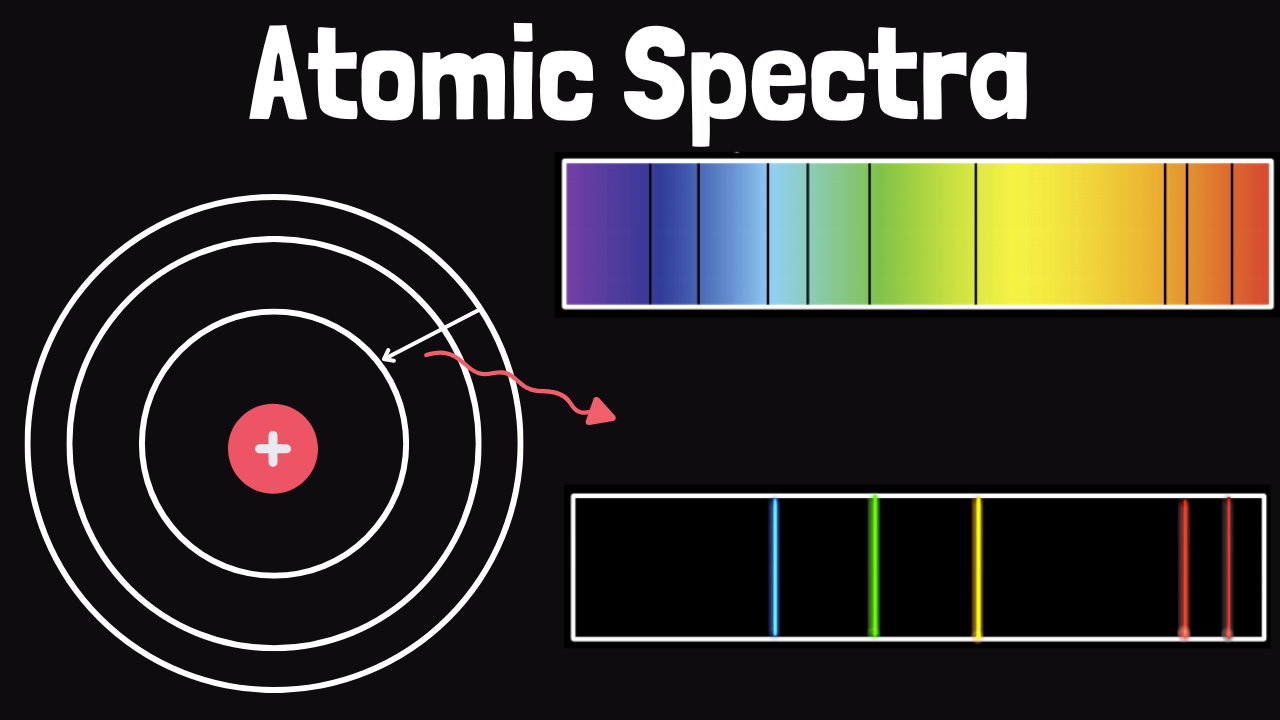
The Quantum Mechanical Model: Unlocking the Secrets of Atomic Emission Spectra
A Fundamental Understanding
To fully grasp atomic emission spectra, it’s essential to have a solid understanding of the quantum mechanical model. This model is based on the principles of wave-particle duality and the uncertainty principle, which were introduced by pioneers like Max Planck, Albert Einstein, and Niels Bohr.
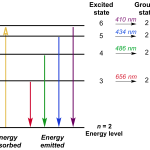
In this model, electrons are thought to exist in specific energy levels or shells around the nucleus. These energy levels are quantized, meaning they can only occupy certain discrete values. When an electron jumps from a higher energy level to a lower one, it releases a specific amount of energy in the form of light.
The Role of Excitation and Ionization
Excitation and ionization play crucial roles in atomic emission spectra. Excitation occurs when an electron absorbs energy and moves to a higher energy level. Ionization happens when an electron is completely removed from its atom, resulting in the formation of ions.
These processes are essential for understanding how atoms emit light. When an atom is excited or ionized, it can release excess energy as photons, which we can observe and analyze.
Key Takeaways
To recap, atomic emission spectra are unique patterns of light that result from the transitions between different energy levels in atoms. The quantum mechanical model provides a fundamental understanding of these processes, revealing how excitation and ionization lead to the release of specific amounts of energy.
In our next section, we’ll explore how scientists use atomic emission spectra to gain insights into the properties and interactions of atoms and molecules.
Learn more about atomic emission spectra on Wikipedia Explore emission spectra in-depth with Physics ClassroomAs we conclude our exploration of 5.3 atomic emission spectra and the quantum mechanical model, let’s summarize the key points we’ve covered so far:
- We discovered that atomic emission spectra are the unique “fingerprints” left by atoms as they transition from a higher energy state to a lower one.
- These spectra reveal the secrets of the quantum mechanical model, providing valuable insights into the properties and interactions of atoms at the atomic level.
- We explored how the quantum mechanical model helps us make sense of these spectral patterns and what they reveal about the behavior of atoms at the atomic level.
So, what can we take away from this journey into the world of atomic emission spectra? First and foremost, it’s a powerful reminder of the wonders that await us when we dive deep into the quantum realm. By embracing the mysteries of atomic emission spectra, we’re not only unlocking new secrets of the universe but also gaining a deeper appreciation for the intricate beauty of the atomic world.
As you gaze up at the night sky or marvel at the glowing lights of a neon sign, remember that these breathtaking displays are just a glimpse into the hidden world of atoms and their emission spectra. And who knows? Perhaps one day, your own curiosity and passion will lead to breakthroughs in fields like chemistry, materials science, or beyond!
In conclusion, our journey through 5.3 atomic emission spectra has been a thrilling adventure that has taken us to the very heart of the quantum mechanical model. As we look to the future, let’s keep exploring, discovering, and marveling at the wonders that await us in this fascinating realm.
The estimating problem on page 734 and then answer the questions on page 735: Ever struggled with estimating? You’re not alone! This article tackles the common pitfalls of estimation and provides a step-by-step guide to help you master this crucial skill. Want to boost your confidence and accuracy in just a few minutes? Click to dive in!
Symptoms of fatty liver due to alcohol consumption: Are you a heavy drinker or know someone who is? This article sheds light on the often-overlooked symptoms of fatty liver disease caused by excessive alcohol intake. Don’t wait until it’s too late – click to learn how to identify and prevent this condition!